Check out 8 Covid-19 vaccines licensed in Vietnam
Currently, the situation of Covid-19 is still complicated. According to the allocation of Ministry of Health, provinces and cities have implemented Covid-19 vaccination for people. Thus, do you know 8 Covid-19 vaccines licensed in Vietnam? This article will provide for you this useful information.
Up to now, there have been 8 Covid-19 vaccines licensed to use in Vietnam by Ministry of Health. Below is some information about these vaccines.
8 Covid-19 vaccines licensed in Vietnam
-
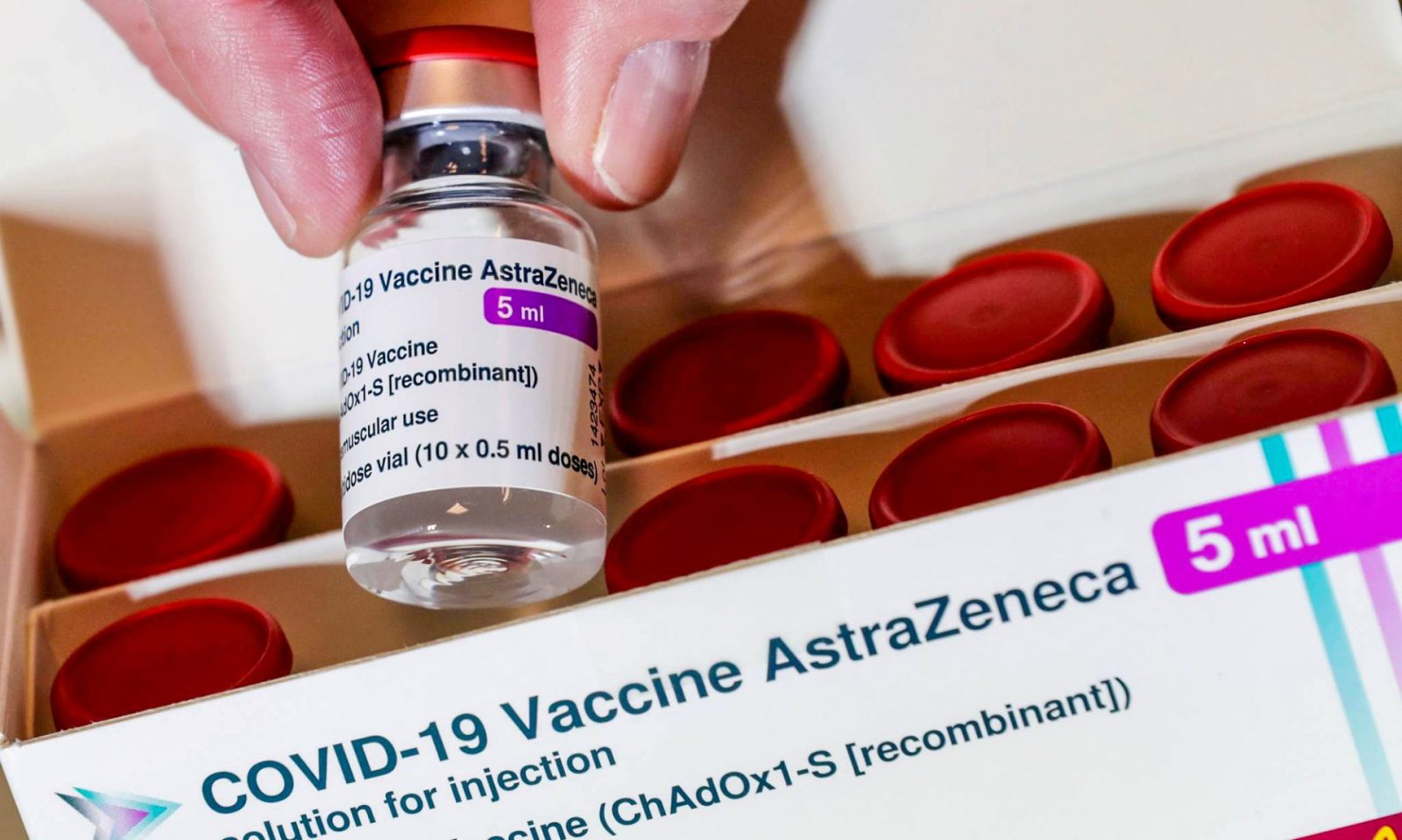
AstraZeneca vaccine
AstraZeneca produced by AstraZeneca Corporation has been licensed for emergency use in 181 countries and territories and has been listed by World Health Organization (WHO) for emergency use. The vaccine was approved on February 01st, 2021 by Vietnam and has been vaccinated since March 2021. Currently, this is also type of vaccine having the largest number of uses in Vietnam. AstraZeneca is produced by vector technology, using 2 doses 8-12 weeks apart.
-
Gam-Covid-Vac vaccine (or Sputnik V)
Gam-Covid-Vac vaccine is manufactured by Gamaleya Research Institute, Russia and has been licensed for use in 70 countries and territories. In Vietnam, Sputnik V vaccine was conditionally licensed for an urgent need in the prevention and control of Covid-19 on March 23rd, 2021 by Ministry of Health. This vaccine uses recombinant technology and carry the gene encoding – Protein S of SARS-CoV-2. The vaccine is injected 2 doses 3 weeks apart.
-
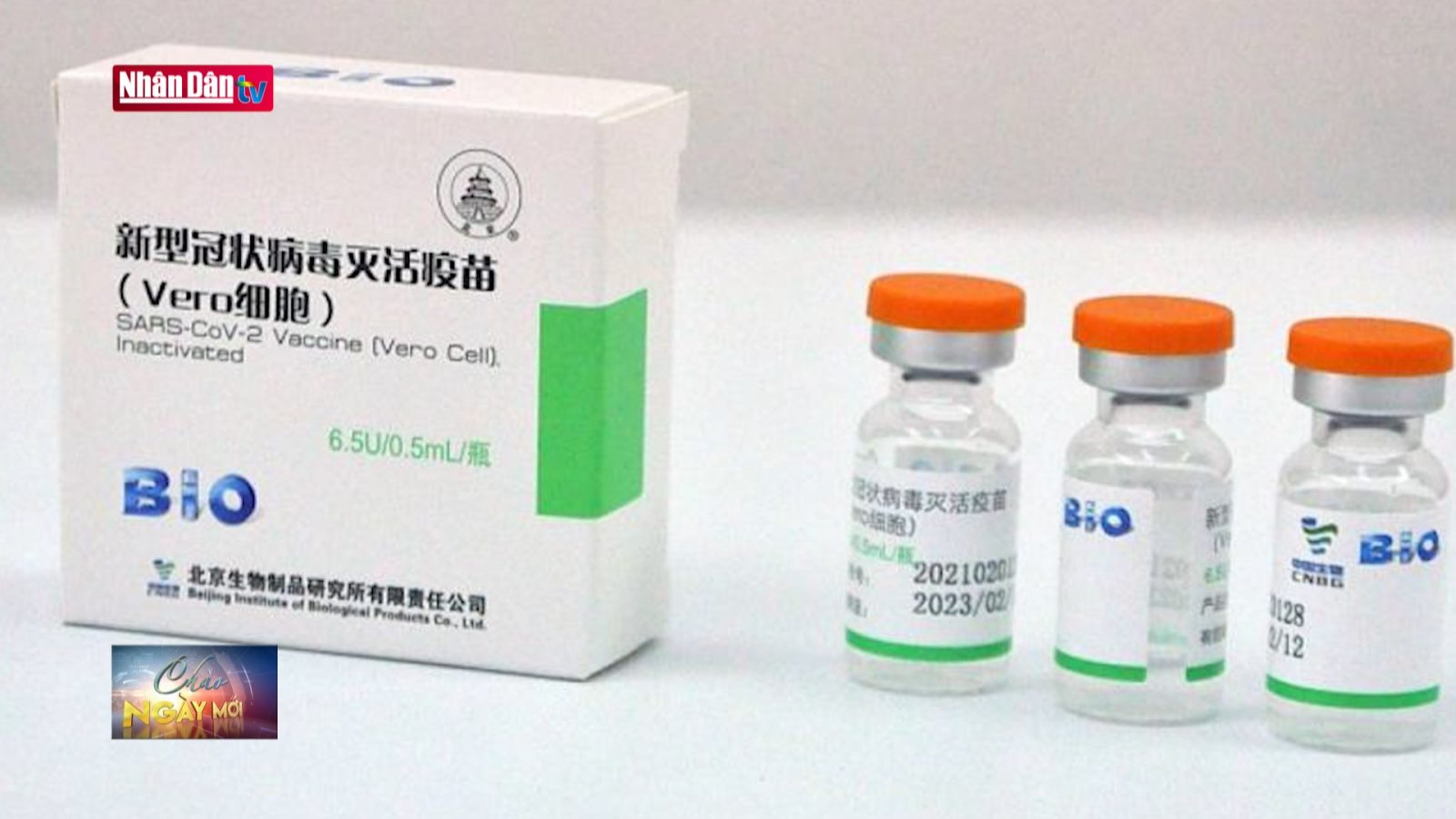
Vero Cell vaccine
Vero Cell vaccine is developed by Sinopharm and Beijing Institute of Biological Products Co. Ltd – China. It has been licensed in 64 countries and territories and has been listed for emergency use by World Health Organization. On June 03rd, 2021, Vero Cell vaccine was conditionally licensed for the urgent need in the Covid-19 prevention and control by Ministry of Health. This vaccine is produced by inactive technology, getting 2 doses 3-4 weeks apart.
-
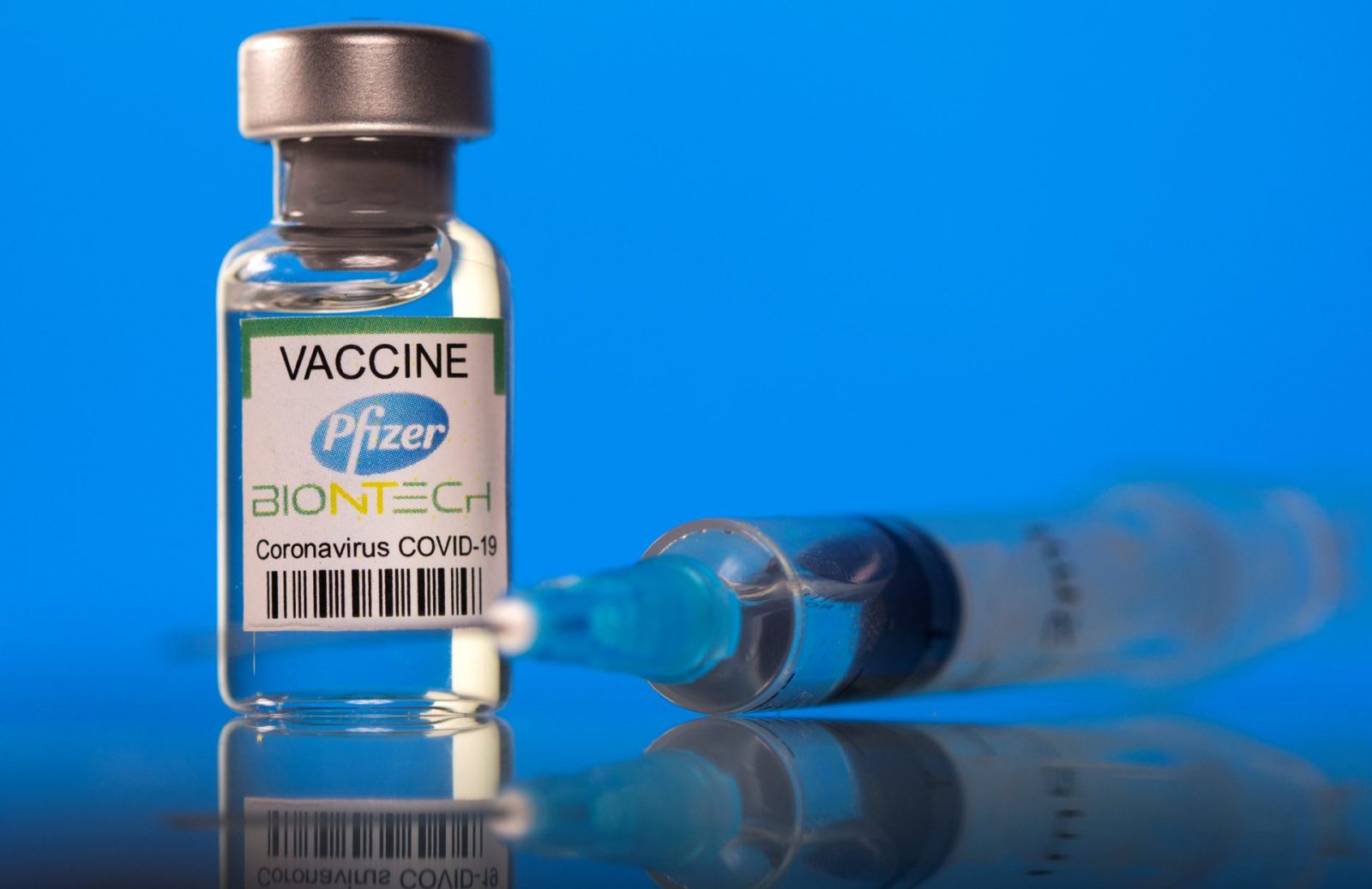
Comirnaty vaccine of Pfizer/BioNTech
Cormirnaty vaccine of Pfizer/BioNTech has been licensed for use in 111 countries and territories and listed for emergency use by World Health Organization. This vaccine was conditionally licensed for the urgent need in the Covid-19 prevention and control on June 16th, 2021 by Ministry of Health. The vaccine is manufactured by mRNA technology, using 2 doses 3-4 weeks apart. Until August 23rd, 2021, US Food and Drug Administration (FDA) officially gave full approval to Pfizer-BioNTech vaccine for the prevention of COVID-19 towards 16 years-old people and older.
-
Spikevax vaccine (or Moderna)
Spikevax vaccine (also known as Moderna) produced by Moderna has been licensed for use in 64 countries and territories and listed for emergency use by World Health Organization. This vaccine is also produced by mRNA technology, using 2 doses 4 weeks apart. Ministry of Health conditionally approved this vaccine for an urgent need in the Covid-19 prevention on June 28th, 2021.
-
Janssen vaccine
Janssen vaccine is produced by Janssen Pharmaceutica NV (Belgium) and Janssen Biologics B.V (Netherlands). It has been licensed for use in 56 countries, territories and listed for emergency use by World Health Organization. The vaccine is manufactured by vector technology, only using 1 dose. Currently, Vietnam has not received this vaccine. However, Ministry of Health conditionally approved this vaccine for the urgent need in Covid-19 prevention and control on July 15th, 2021.
-
Hayat – Vax vaccine
Hayat – Vax vaccine is manufactured in semi-finished products by China National Biotec Group (CNBG). This vaccine is packaged in primary, secondary and shipped at Julphar (Gulf Pharmaceutical Industries) – United Arab Emirates. Each 0.5 ml dose of Hayat – Vax vaccine contains 6.5 SARS-CoV-2 inactive antigens (vero cells), prepared in suspension for injection. The vaccine is packaged in a box of one vial containing 1 dose of 0.5 ml and a box of one vial containing 2 doses, each dose of 0.5 ml. Ministry of Health issued a conditional approval decision for this vaccine on September 10th, 2021 for an urgent need in Covid-19 prevention and control. The dose of injection is waiting for Ministry of Health to update the guidelines.
-
Abdala vaccine
Abdala vaccine is manufactured in finished products at AICA Laboraries, Base Business Unit (BBU) – Cuba and exported semi-finished products, secondary packaged at the Center for Genetic Engineering and Biotechnology (CIGB) – Cuba. Each 0.5 ml dose of Adbala vaccine comprises 50 mcg of recombinant protein vaccine containing SARS-CoV-2 receptor-binding region (RBG), prepared in suspension for intramuscular injection. The vaccine is packaged in a box of 10 vials, each vial containing 10 doses, each dose of 0.5 ml. Ministry of Health conditionally approved for an urgent need in Covid-19 prevention and control towards this vaccine on September 17th, 2021. The dose of injection is waiting for Ministry of Health to update the guidelines.
Above is the whole information of 8 Covid-19 vaccines licensed in Vietnam. Surely, you have had a deeper understanding about the vaccines and its relevant information. In addition to getting vaccination, we also need to well-implement the guidelines of Ministry of Health. As long as we try together, we will soon defeat the pandemic.
(Reference source: moh.gov.vn)
Some articles you may concern: